Abstract

Background
Chronic iron overload is an important complication of long-term blood transfusions for severe beta-thalassemias, sickle cell disease and other blood disorders.
Iron chelation therapy (ICT) is required to bind and excrete excess iron, which would otherwise accumulate and lead to organ damage or failure.
Deferasirox is a once-daily, orally administered ICT approved for the treatment of chronic iron overload due to frequent blood transfusions in patients with beta-thalassemia major and other anaemias. A film-coated tablet (FCT) formulation was launched in the UK in 2016 and replaced the dispersible tablet (DT) formulation.
In the context of a randomised clinical trial, the FCT formulation showed greater adherence and patient satisfaction, better palatability and fewer tolerability concerns than the DT. Furthermore, treatment compliance by pill count was higher with FCT (92.9%) than with DT (85.3%) (Taher et al, 2017). Little information exists however about compliance, efficacy and tolerability outside of a clinical trial setting.
Objectives
We wished to assess in a 'real world' situation, the effects of switching the deferasirox formulation from DT to FCT on patient adherence to ICT, iron overload and renal function.
Methods
Patients receiving ICT with deferasirox who were switched from the DT to FCT formulations were followed over a 12-month period and results audited using hospital dispensing and biochemistry records. The date of the first FCT prescription was defined as baseline.
The initial daily dose used for switching from DT to FCT was as per manufacturer's recommendations: 70% of the DT daily dose.
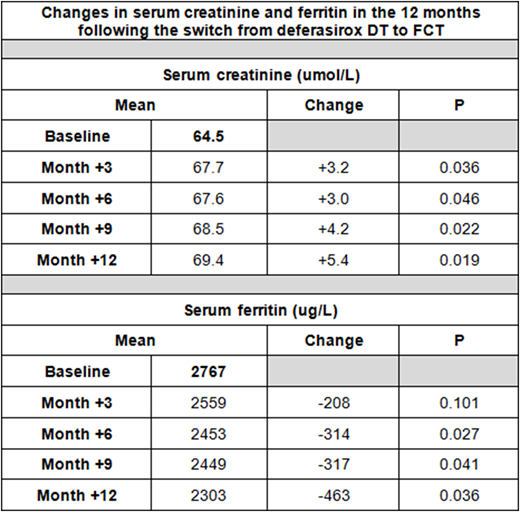
The impact on iron overload was assessed by comparing serum ferritin levels at 3, 6, 9 and 12 months post-switch with baseline values. The impact on renal function was assessed by comparing serum creatinine levels at 3, 6, 9 and 12 months post-switch with baseline values as well as the number of serum creatinine increases of 30% or greater above baseline.
The changes in serum ferritin and creatinine were subsequently analysed by paired t-test.
The Proportion of Days Covered (PDC) was calculated as a measure of patient adherence to ICT in the 12 months before and after switching formulations.
Results
74 patients switched from deferasirox DT to FCT with the following diagnoses: beta-thalassemia (n = 45), sickle-cell disease (9), thalassemia-intermedia (6), HbE-thalassemia (5), other transfusion-dependent disorders (9). The median age was 36 (range: 1-78yo), mean baseline serum ferritin was 2767µg/L (range: 412-8742), mean baseline creatinine was 64.5 umol/L (range: 17-140) and the median prescribed daily dose of DT was 1250mg (range: 62.5 - 3500).
The mean PDC in the 12 months prior to switching formulations was 0.80 (range: 0.31-1.00). This increased to 0.91 (range: 0.21-1.00) in the 12 months following the switch to FCT. The median prescribed daily dose of FCT was 900mg (range: 90 - 2520)
The mean changes in ferritin and creatinine at 3, 6, 9 and 12 months post-switch are shown in the table.
6 out of 74 patients (8%) had a creatinine increase of >30% from baseline whilst receiving the FCT, occurring after an average of 120 days (range: 30-260). All 6 patients were managed by dose adjustment of FCT and creatinine returned to the normal range in 5 out of 6 cases.
Conclusions
The switch from deferasirox DT to FCT resulted in improved patient adherence to chelation, a reduction in mean serum ferritin and a modest rise in mean serum creatinine. Some patients showed a reversible rise in creatinine from baseline.
The median daily dose of FCT prescribed was 72% of the DT formulation, approximately equivalent according to the known bioavailability of the different preparations and suggesting that improvements in serum ferritin were due to the more consistent daily administration of the FCT rather than an increased daily deferasirox dose.
We suggest that when the fall in ferritin is abrupt and/or to levels <1000µg/L, serum creatinine should be followed particularly carefully to avoid over-exposure to deferasirox from the FCT. We further speculate that patients who may have over-reported adherence to the DT prior to switching may be most susceptible to this effect.
Reference
Taher, A. T., et al. (2017). "New film-coated tablet formulation of deferasirox is well tolerated in patients with thalassemia or lower-risk MDS: Results of the randomized, phase II ECLIPSE study." American journal of hematology 92(5): 420-428.
Garbowski:Vifor: Consultancy. Porter:Novartis: Consultancy; Cerus: Honoraria; Agios: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal